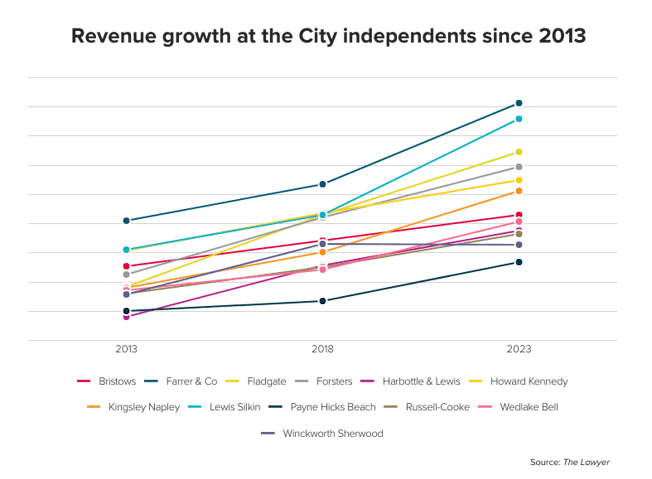
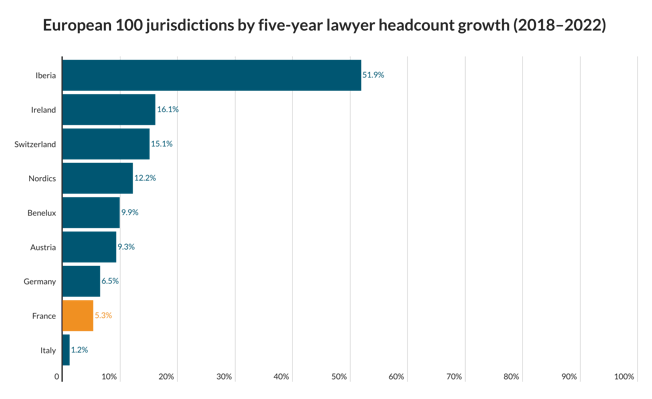
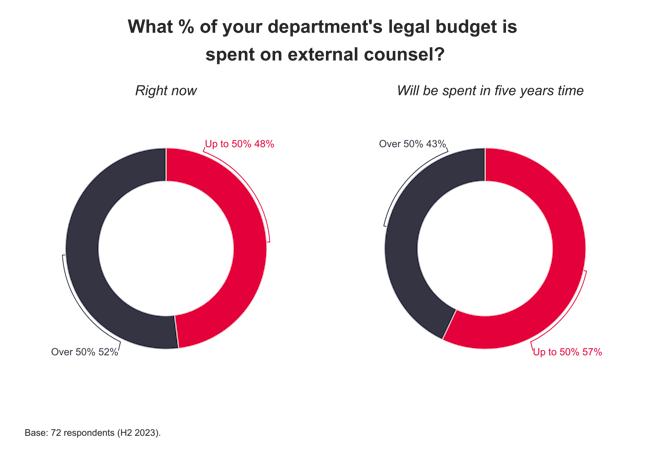
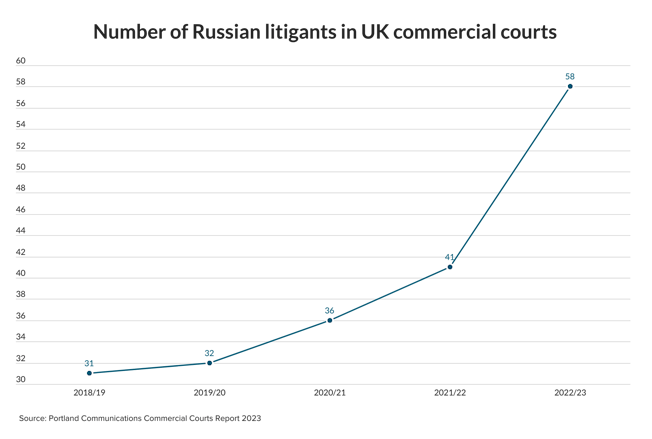
The pharmaceutical industry is worth billions of dollars and many law firms have pharma clients. If you’re interested in working for one of those firms, here’s what you need to know about what’s currently going on in the sector.
The Association of British Pharmaceutical Industry estimated in 2014 that the average cost of bringing a prescription drug through clinical trials to final approval was approximately £1.2bn and that it took on average 12 years. With such vast sums being expended on the development of new drugs it may perhaps be surprising to know that, according to Forbes Magazine, 95 per cent of experimental medicines studied in humans fail to secure approval either because they are unsafe, ineffective or a combination of both.
However, those drugs that do secure approval and go on to become blockbuster drugs, such as the top selling drug of 2014, Humira, can bring in global sales of approximately £6bn a year. Recently, though, the organisations that pay for medicines, such as the NHS and insurers, are increasing their focus on the cost of medicines and putting pressure on the margins that the industry can achieve for even its most successful drugs.
The fulfilment of regulatory requirements needed to gain approval from the relevant medicines authority accounts for a significant part of the costs of drug innovation. However, once approved, the innovator company then has a period of approximately 10 years remaining of their patent monopoly in which to exploit the innovation and recover research and development costs before the medicine goes off-patent.
The Patent Cliff and Generic Therapeutics
Going off-patent, colloquially termed the Patent Cliff, marks the steep decline in revenue that drug innovators face when their patent protection ends. The loss in sales may run into the billions as a result of loss of market share, competition with generic manufacturers, and a race to the bottom in relation to pricing practices as more generics gain market approval.
In order to extend the period of patent protection and defend their revenues, innovators typically file for patent protection not just on the compound itself but also on methods of formulating the medicine, dosages and treatment of new diseases; so called secondary patents. Generic companies generally respect the patents on the compound but will bring court proceedings to attempt to invalidate secondary patents and therefore get onto the market with their generic versions.
As well as defending the patents on their existing medicines, drug innovators need to renew their pipelines of new drugs by in-licensing new medicines (mainly at an early stage of development) or acquiring or merging with other drug innovators
This issue is not solely limited to innovators of small molecule drugs. Innovators of biologics are equally concerned with the impact of biosimilars on their market share and sales revenue. In such circumstances, the old adage rings true: if you can beat them, join them. Companies such as Amgen, Pfizer and Novartis are in various stages of making their own biosimilar equivalents to well-known biologics such as Herceptin and Avastin.
The role of the lawyer
As the innovator industry continues to defend patents on its existing medicines and acquire new medicines to bolster its pipelines, the role of the lawyer cannot be understated. Pharmaceutical sector lawyers will be involved in a variety of roles including patent litigation, drafting licensing and collaboration agreements between small biotech companies and “Big Pharma” or acting in mergers or acquisitions.
The legal professional has to be familiar with the specialised pharmaceutical regulatory environment, the intellectual property system, and the commercial pressures on the industry as well as gaining an understanding of the underlying science behind their client’s medicines. It is an exciting time to be in this sector of law.
Elwin Morgan is an associate at Wragge Lawrence Graham & Co
Further reading…