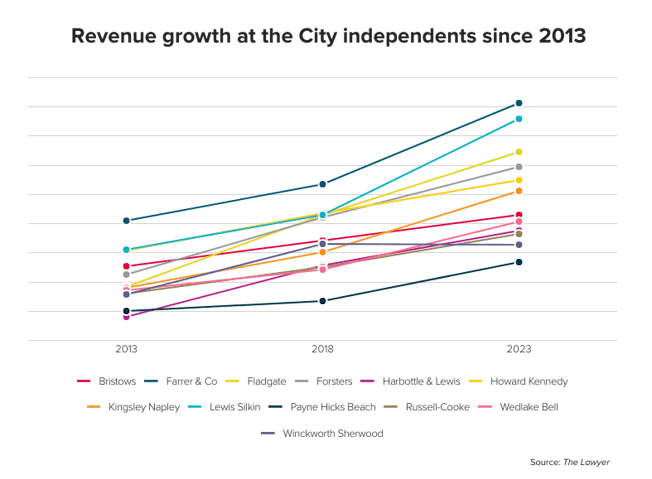
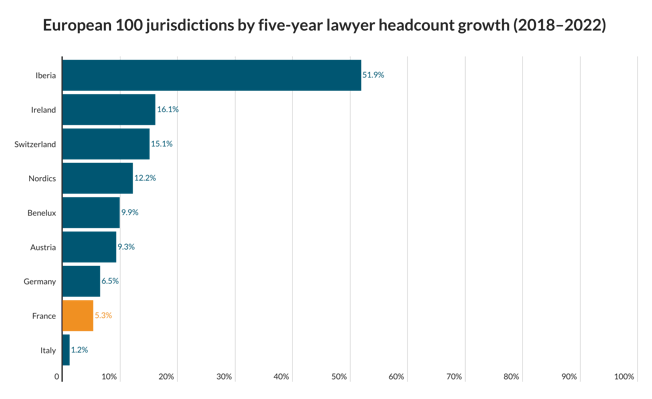
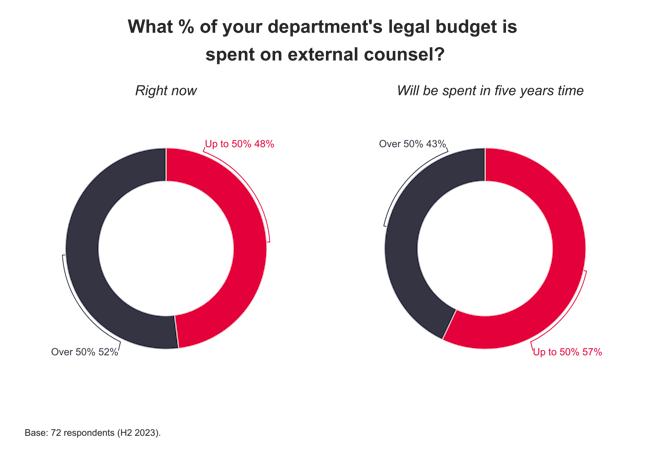
Erskine Chambers seals victory for RBS in Liverpool FC dispute
Mr Justice Floyd has granted the Royal Bank of Scotland (RBS) mandatory injunctive relief against Liverpool Football Club, paving the way for the sale of the club. The bank went to the High Court in a bid to force the club’s owners Tom Hicks and George Gillett to reconstitute the club’s board and allow it […]