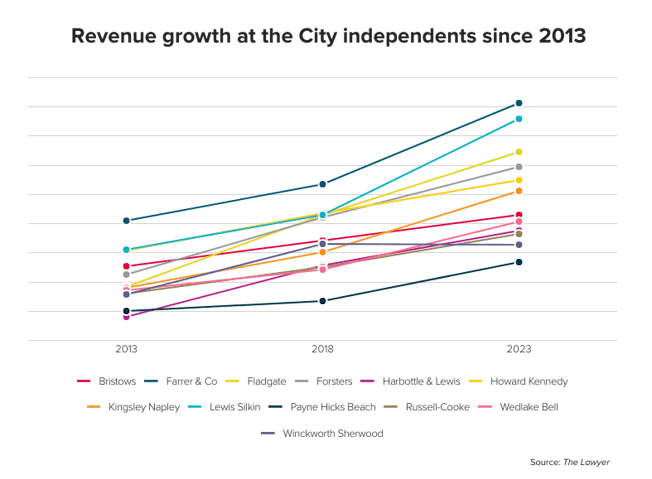
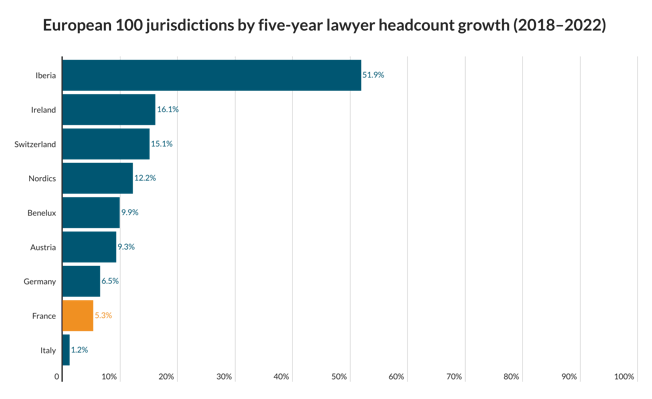
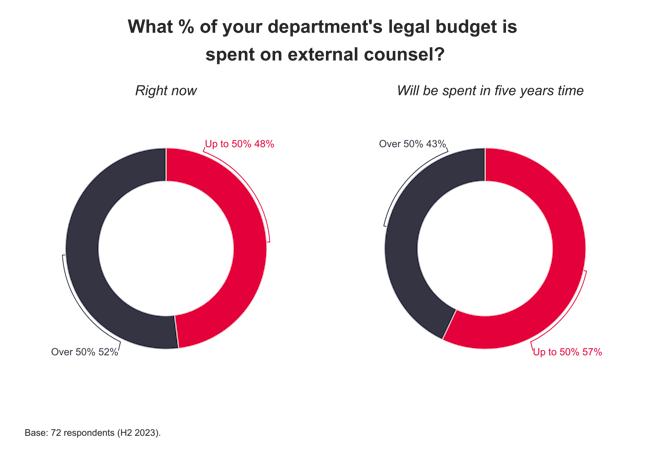
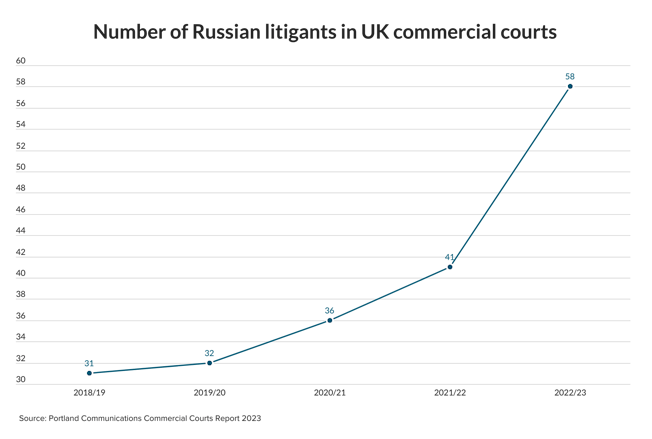
A legal battle over the right to market a product aimed at keeping teeth white is on its way to the Law Lords. Lords Hope, Browne-Wilkinson and Hutton have given leave for Optident to challenge Appeal Court backing for moves by the Secretary of State for Trade and Industry which it says amount to unlawful obstruction of its marketing programme. The company claims that Opalescence, the product at the centre of the battle, is "a medical device" and in Germany has been licensed with a CE mark under the Medical Devices Directive 93/42/EEC. But the Secretary of State has taken the view that under the EEC Cosmetics Directive it is a cosmetic. The legal status of the product dictates the level of hydrogen peroxide it can contain. The House of Lords is to decide whether a CE mark obtained in one EC member state can be over-ruled by another. The Lords will also be asked to decide the true legal status of Opalescence as a "cosmetic" or "medical" device.
E-commerce boom aids TJG expansion
Taylor Joynson Garrett has expanded its private client department on the back of the boom in electronic commerce and the new economy. Mark Buzzoni joins the firm as a partner, leaving Alexanders, the trust and litigation specialist based in Temple. TJG managing partner Declan Tarpey says the appointment reflects the amount of wealth created by […]