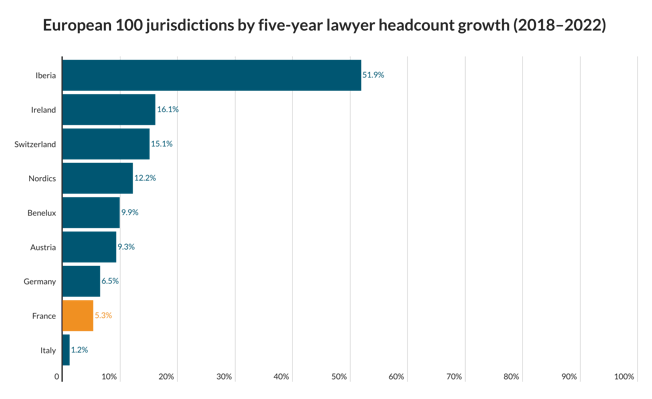
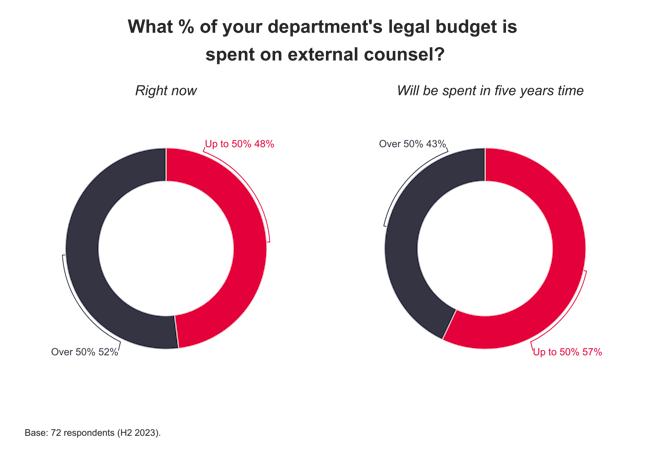
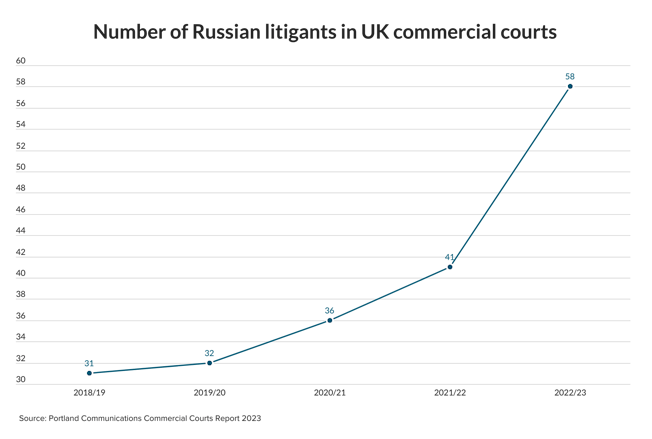
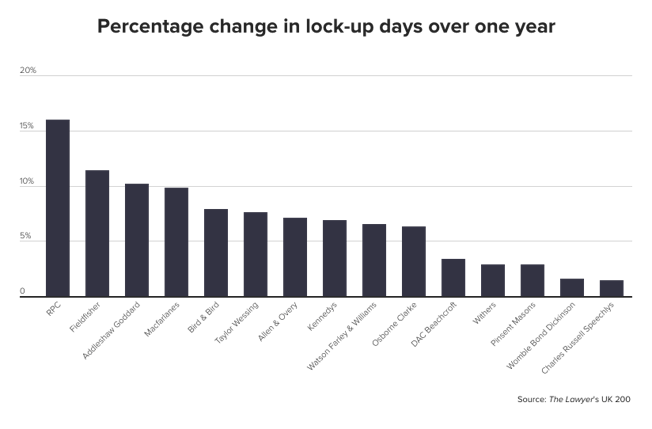
M&A star Ashworth quits Ashurst ahead of new partnership deed
Ashurst corporate heavyweight Chris Ashworth is leaving the silver circle firm ahead of a new partnership agreement. The firm was informed by email this afternoon of his decision to resign after 23 years of practice. Ashworth stepped down from the post of head of corporate two years ago. During his time at the firm has […]